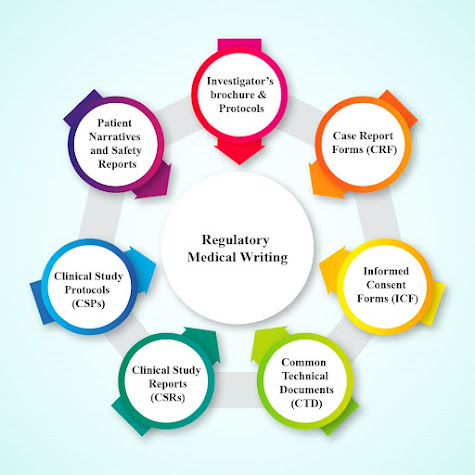
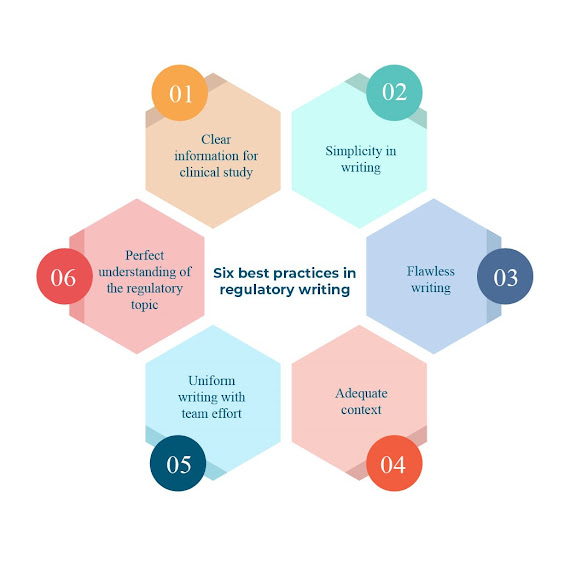
Regulatory Submission An adverse event is reported and filed when a healthy volunteer/patient has a negative reaction to a drug. It contains information about the drug, its dose, purported effect, the patient’s health before and after the adverse event, and the patient reactions to the drug before and after the adverse event are among other things. Adverse event reporting sources include the information on the adverse event gathered from many sources. The types of reports include spontaneous/voluntary reports, clinical trials and post-marketing studies, regulatory reports, license partner reports, and literature reports. Regulatory authorities reviews the results of laboratory, animal, and human clinical testing done by companies for pre-marketed products before approving worldwide. They monitor the benefit/risk balance of marketed drugs, in accordance with each country’s local or regional laws and regulations. #regulatorycompliance #regulatoryreporting #regul...