Regulatory Affairs Management
WorkSure®
Regulatory Affairs is a collaborative science which enhances
comprehensive drug/medical devices/technology development journey. We navigate
regulatory complexity and bring your innovations to patients safely and efficiently.
Our Regulatory Affairs team ensure correct data analytics to optimize
submission timelines. #TeamWork #Clinicalresearch #RegulatoryAffairsTeam.
Watch at: https://we.tl/t-Y9vcSrqBsV

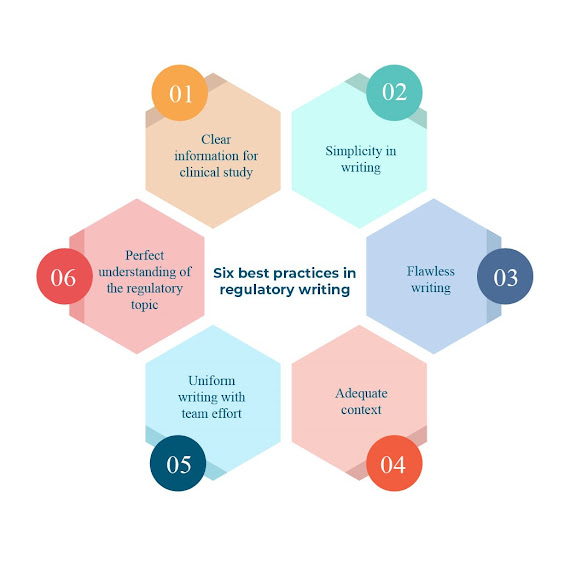

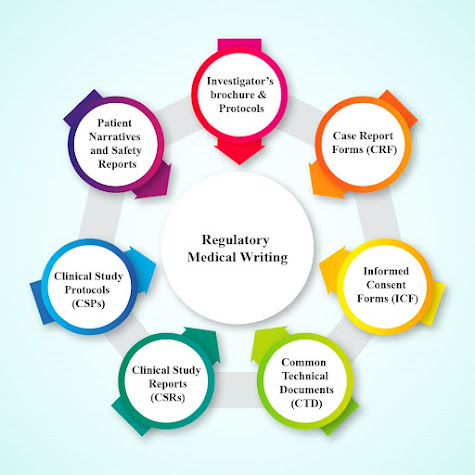
Comments
Post a Comment