REGULATORY WRITING – VITAL FOR CLINICAL STUDY
Regulatory
writing, a branch of medical writing, is essential for the conduction of
clinical trials approved by the higher regulatory authorities. Regulatory
medical writing encompasses various clinical documents produced during the
various phases of treatment starting from description and documentation of the
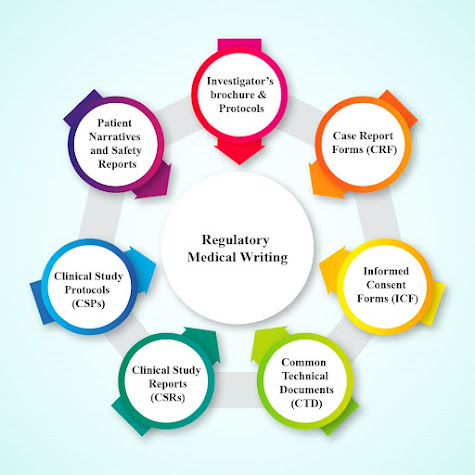
clinical trials to preparation of the regulatory suggestion documents. Examples
of clinical documents applied in regulatory writing are the Common Technical
Documents (CTD), Clinical Study Reports (CSRs), Clinical Study Protocols (CSPs),
Patient Narratives and Safety Reports.
The
important national regulatory authorities are:
·
Central
Drugs Standard Control organization (CDSCO) (India)
·
Food
and Drug Administration (FDA) (United States)
·
Health
Canada (Canada)
·
European
Medicines agency (EMA) (European Union)
·
Ministry
of Health, Labour and Welfare Japan (Japan)
The efficacy and the safety of the drugs for overall clinical trial processes are assessed by each authority and approve the investigative drug or the medical device or the use of the compound accordingly.
Functions of the regulatory
writer
Regulatory
writing includes the experts from clinical or medical field, statisticians and
persons from regulatory affairs, pharmacovigilance and pharmacology. Regulatory
writing also includes quality control (QC), peer review or editorial support of
the documents provided by the study team or the sponsors. The core skill set of
regulatory writing requires the proper understanding of the target audience and
regulation impairment, knowledge of statistics, research, study design, project
management, explicating key messages, operating different software,
collaborative skills to work with reviewers and possess ethical standards. It is essential to understand,
interpret and summarize the complex scientific and statistical data and provide
effective guidance to clinical study teams.
The
job is to transform the huge amount of the clinical information administered
during the clinical preliminaries into clear and accurate records for effective
support to all the administrative specialists.
A standard layout is very much essential for the clinical author in
order to maintain perfect harmony between phrasing and harmony of different
sectors of documentation. Effective and efficient team work leads to a better
documentation.
Key points for the
regulatory medical writer
The
regulatory medical writer is majorly responsible for documenting the complete
cycle of the clinical study. The writer has to be updated of the latest
regulations and amendments of different countries to give a clear view of the
study. The regulatory documents have to be written in such a manner that it is
transparent, accurate and easily transmittable to the target audience. The target
selection depends on the type of document such as regulatory authorities for eCTD
modules, patients for ICF, and trial investigators and ethics committee for
Protocol, IB and CSR.
Importance of regulatory
writer
In the dynamic regulatory environment,
experienced regulatory writers can enhance the quality of the clinical study
documentation. At every step, medical writer can prove to be knowledgeable
playing a fundamental role in the clinical research process. Regulatory writers
are efficient in meeting global regulatory requirements and assist in guiding
the documentation such as International Conference on Harmonisation (ICH), Good
Clinical Practice (GCP) as well as providing full support for the needs,
preferences and styles of the sponsors or the team.
Regulatory Writing at Worksure®
At Worksure®, we produce clinical documents that are required for smooth
conduct of clinical studies (encompassing the complete cycle of Phase I, Phase
II and Phase III clinical trials) as well as clinical monitoring. The documents
are prepared depending on the three stages of the trial mainly:
·
Pre-clinical trial stage
·
During the clinical
trial
·
Post-clinical trial
stage
Investigator’s brochure, protocols (with detailed technical/laboratory/medical procedures and safety reporting) and amendments, Case Report Forms (CRF), ICF, documented approval of Institutional Review Board (IRB) or Independent Ethics Committee are the crucial documents generated before the onset of the trial. A regular update of the above-mentioned documents along with monitoring visit reports, subject screening and enrolment log, record of retained body fluids or tissue samples, and other documents are essential during clinical trial conduct. Additionally, documents prepared after the conduct of clinical trial encompass the finished subject identification code list, the final trial close-out monitoring report the investigator’s final report to the IRB/IEC, and the Clinical Study Report, which contains the trial’s data and interpretation. A well-documented CSR producing superior quality papers is a requisite to get approval from the regulatory authority. After regular review of the investigator/institution, the sponsor files and the essential paperwork, the monitor official declares the closure of the trial.

Comments
Post a Comment